VIVAN Life Sciences

We are a Research driven, innovative high-end small quantity Phrmaceutical Research Product synthesis & development Company.
Our Offerings would enhance the capability and productivity performances of our Clients around the globe, in functions critical to their success, which involves greater degree of competency and technological prowess.
We are a unique partner to our Clientele, in providing best possible high-end synthesis products & service requirements, in their critical functions and transformational projects.
As a Global Enterprise, we stand to lead our Business operations, through innovation, creativity and progressiveness with methods, technologies and operating practices.
Quality Deliverance at par with Global Standards, enabled us to provide, exceptional value to our Clients from various Business verticals, there by deriving 'Superior Client Satisfaction'.
Read More

Worldwide Shipping

Quality And Confidentiality

Catering To VariousIndustrial Verticals

High EndCustom Synthesis
Recently Added Compounds









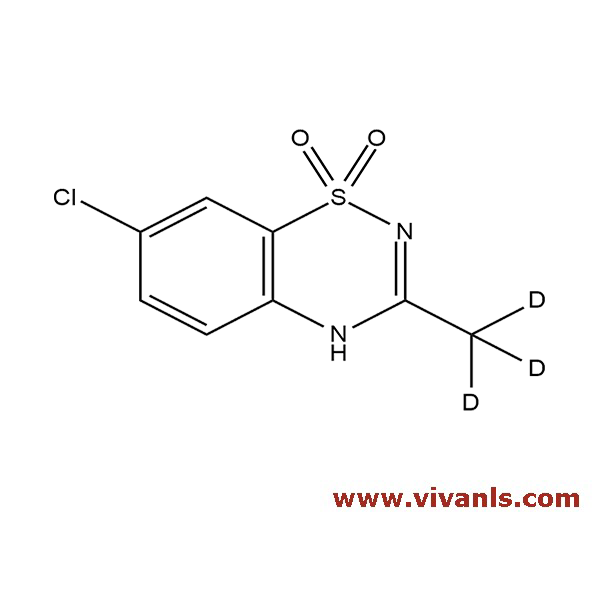
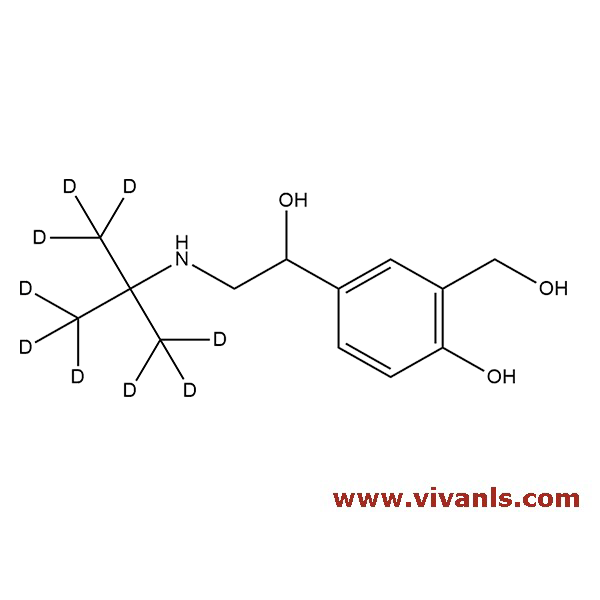
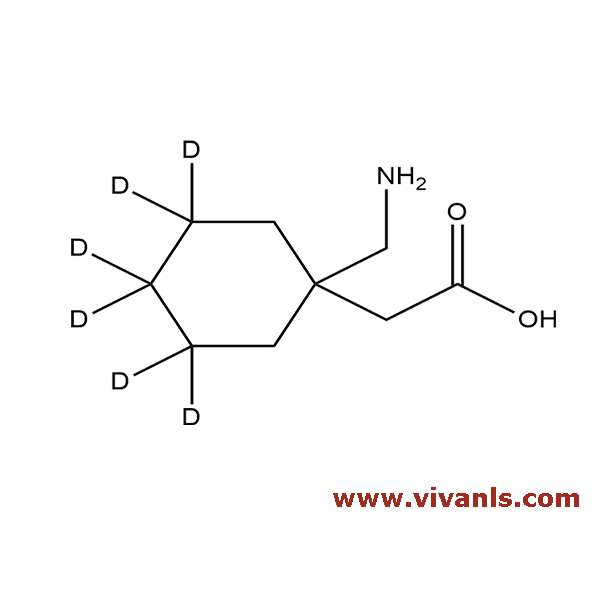
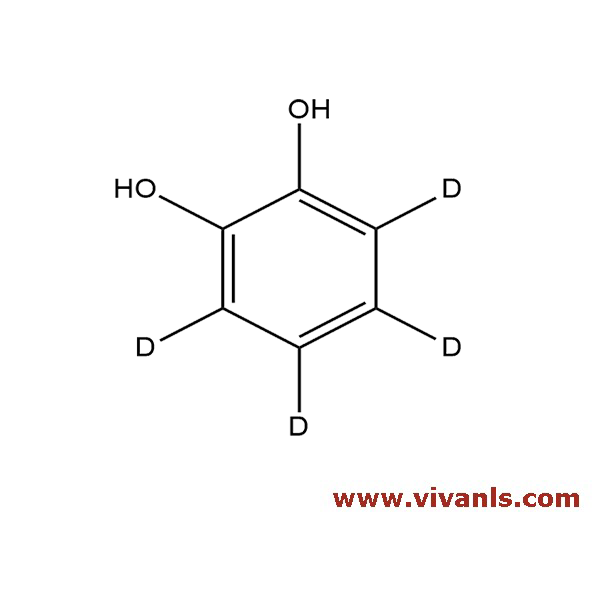
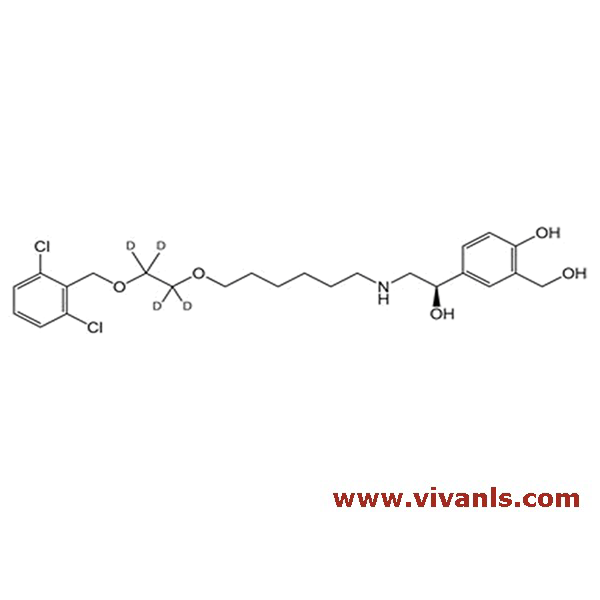
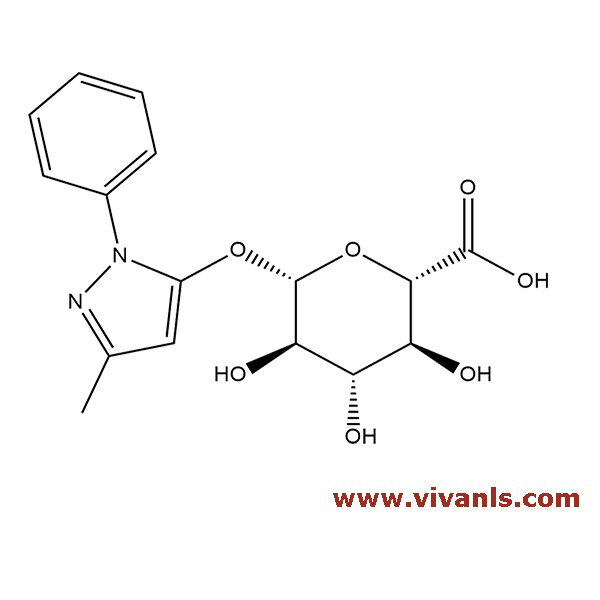
VIVAN is trusted by many Global Enterprises and innovative SME enterprises working on Cutting Edge Technologies alike, for over a decade. We cater to various Business fields, though our innovative products in high-performance Materials, high-end Chemicals and Pharmaceutical Research. Just to mention, we have all top 10 Pharmaceutical companies in INDIA as our trusted Clientele.
"We Take Pride In Our Work"
-
@VIVAN Life Sciences we offer small quantity high-end Pharmaceutical Working standards, Reference materials and Product Synthesis & Development, with Global Quality Standards.
-
We enable our Global Clientele, to have a greater value proposition for their Business, by substantially reducing their R&D efforts and Time.
👍 Just to mention All TOP 10 Pharmaceutical Companies in INDIA are our clientele, for the past 13 Years, we take pride in it.
-
We @VIVAN Life Sciences, constantly put efforts in learning and improving newer Technologies in Product Synthesis, This has enabled us to be a TRUSTED R&D Partner to our clientele around the Globe.
-
Our efforts in Advanced Synthesis of small quantity high-end products & Standards, enabled our Global & Local Clientele to derive productivity performances on their critical projects.
-
VIVAN Life Sciences, brings in unique proposition of immense value addition through our capabilities to our Clientele, We can be a great R&D partner for your high-end small quantity product requirements.

Latest Industry News and Information
- To know the latest Industry News and Information.
- Updates on technological evolutions in Pharma & Life Sciences.
- Interesting story snippets for better contextual understanding.
- Short stories on Management & Leadership.
- Last but not the least, We welcome your participation.
- This certainly helps us to better the content sharing on VIVAN BLOG


















