VIVAN Life Sciences Offers wide range of Impurities / Pharmaceutical Drug Impurities. Impurities are unwanted chemicals that remain with the active pharmaceutical ingredients (APIs) or develop during formulation or upon aging of both API and formulation. The presence of these unwanted chemicals even in trace amount may influence the efficacy and safety of pharmaceutical product. The control of impurities is an important task pharmaceutical impurities as per the regulatory norms. High Pure and Well characterized impurity Standards are used for Related Substances, Organic impurities and Validation of Analytical Methods.
All the Impurity standards are provided with CoA, MASS, NMR & HPLC reports, conforms to Global quality standards.

VLIM-00001
1391053-95-4
C₁₇H₂₀N₄O₅S
392.43

VLIM-00002
59365-66-1
C₁₁H₁₆O₃
196.3

VLIM-00003
108-68-9
C₈H₁₀O
122.2
-1,2-Propane Diol(Impurity -A)-1664172015.png)
VLIM-00004
59365-66-1
C₁₁H₁₆O₃
196.246

VLIM-00005
76801-85-9
C₃₇H₇₀N₂O₁₂
734.96
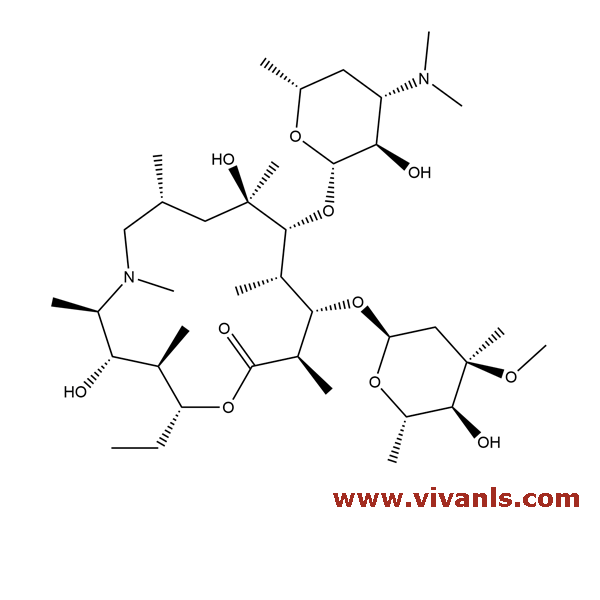
VLIM-00006
307974-61-4
C₃₈H₇₂N₂O₁₁
732.98

VLIM-00007
612069-27-9
C₃₆H₆₈N₂O₁₂
720.5
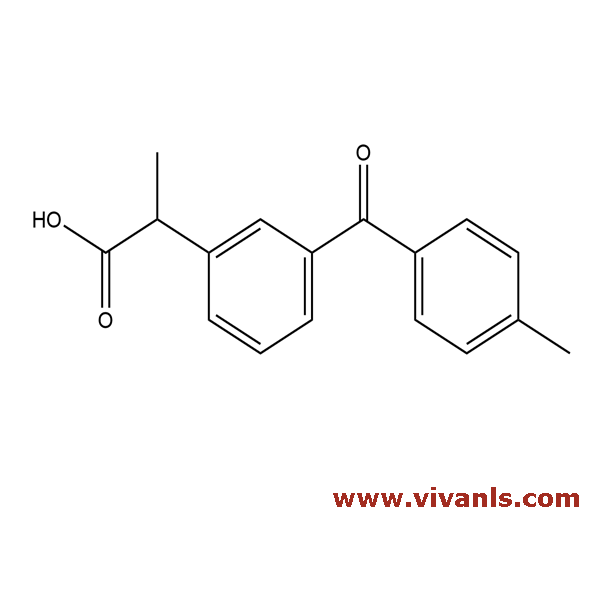
VLIM-00008
107257-20-5
C₁₇H₁₆O₃
268.31

VLIM-00009
151006-14-3
C₃₁H₅₅NO₇
553.77
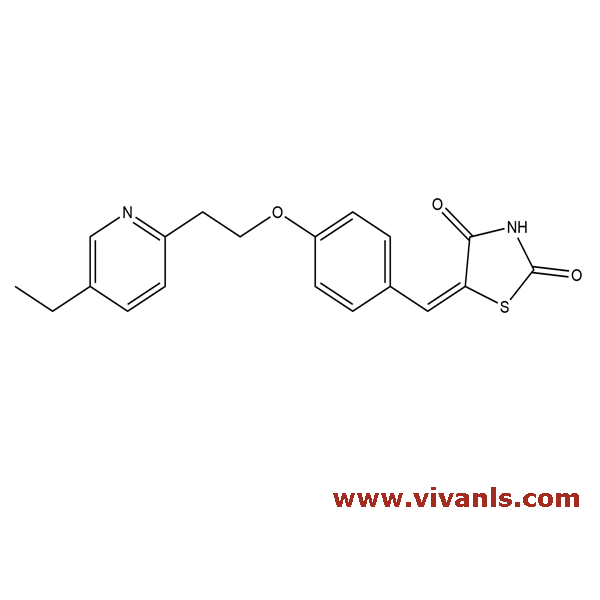
VLIM-00010
144809-28-9
C₁₉H₁₈N₂O₃S
354.423

VLIM-00011
7519-36-0
C₅H₈N₂O₃
144.13
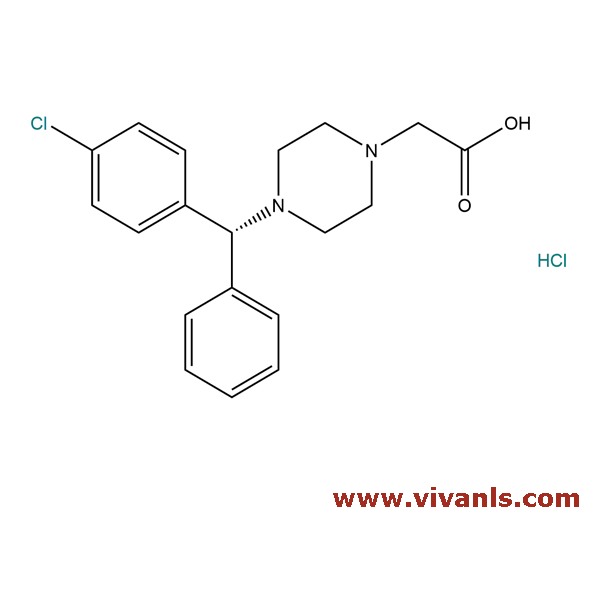
VLIM-00012
942132-30-1
C₁₇H₁₉ClN₂
286.8