VIVAN Life Sciences Offers wide range of Impurities / Pharmaceutical Drug Impurities. Impurities are unwanted chemicals that remain with the active pharmaceutical ingredients (APIs) or develop during formulation or upon aging of both API and formulation. The presence of these unwanted chemicals even in trace amount may influence the efficacy and safety of pharmaceutical product. The control of impurities is an important task pharmaceutical impurities as per the regulatory norms. High Pure and Well characterized impurity Standards are used for Related Substances, Organic impurities and Validation of Analytical Methods.
All the Impurity standards are provided with CoA, MASS, NMR & HPLC reports, conforms to Global quality standards.

VLIM-00013
252964-65-1
C₃₅H₃₈Cl₂N₈O₄
778.85

VLIM-00014
90503-06-03
C₃₈H₇₂N₂O₁₃
764.98
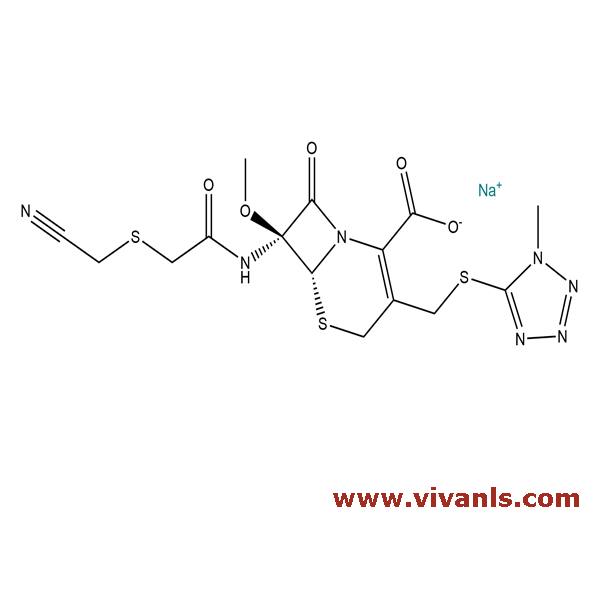
VLIM-00015
56796-39-5
C₁₅H₁₆N₇NaO₅S₃
493.51

VLIM-00016
54029-12-8
C₁₂H₁₅N₃O₃S
281.33
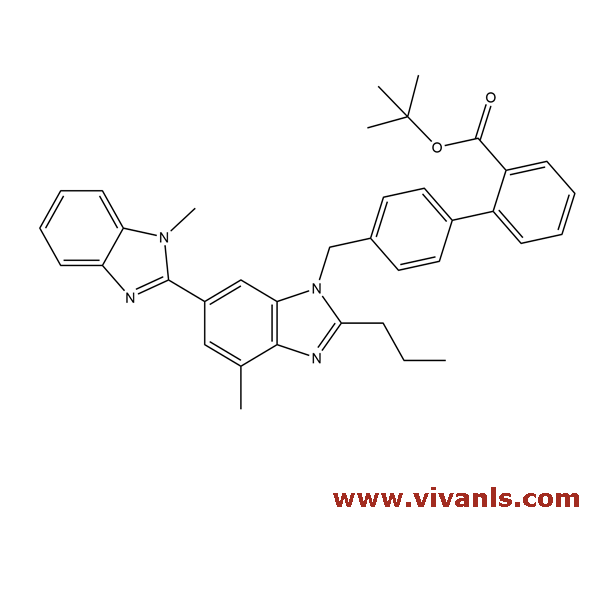
VLIM-00017
144702-26-1
C₃₇H₃₈N₄O₂
570.72
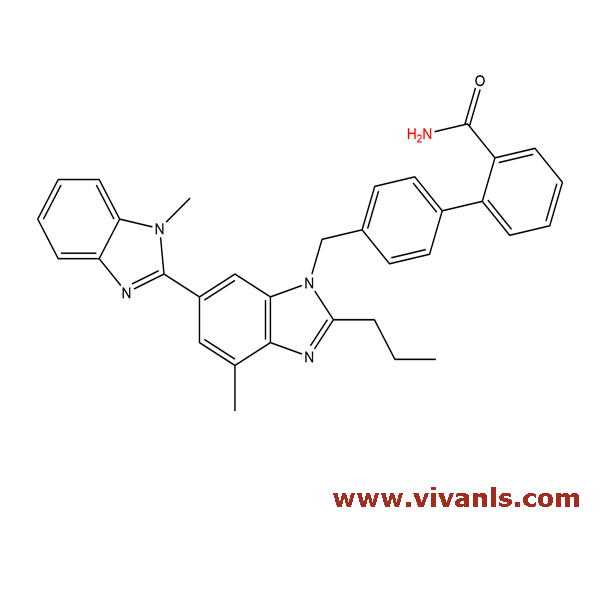
VLIM-00019
915124-86-6
C₃₃H₃₁N₅O
513.63

VLIM-00020
109461-15-6
C₁₇H₁₆N₂O₂
280.32
 Sitagliptin Mandalate Acid-1664172875.png)
VLIM-00021
486460-00-8
C₈H₈O₃
152.15

VLIM-00022
1012-05-01
C₁₀H₁₃NO₂
179.22
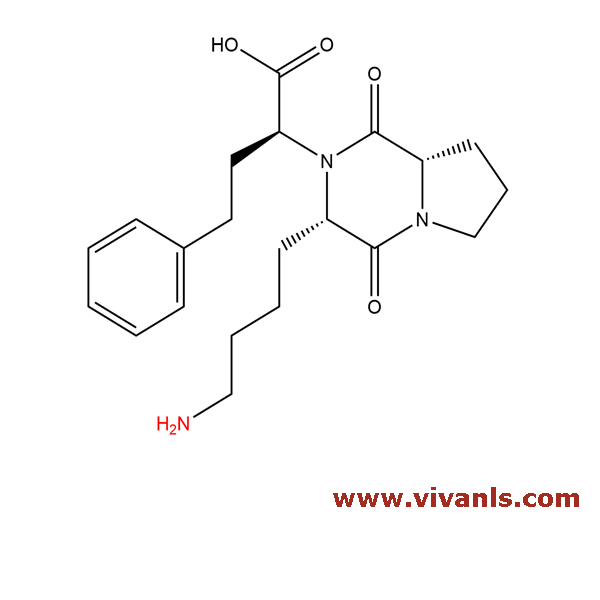
VLIM-00023
328385-86-0
C₂₁H₂₉N₃O₄
387.47

VLIM-00024
219677-82-4
C₂₁H₂₉N₃O₄
387.47
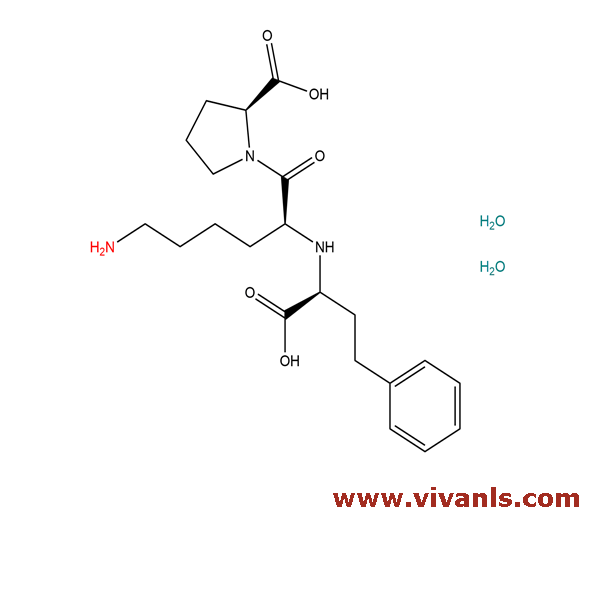
VLIM-00025
83915-83-7
C₂₁H₃₅N₃O₇
441.52